Synthetic methanol is a chemical substance, one of the main raw materials for the organic chemical industry. There are many ways to produce methanol in industry, such as methanol from natural gas and methanol from oil.
Development of Methanol Synthesis

The early production of methanol was obtained by dry distillation of wood. After the development of the synthetic method, this method was almost eliminated. The initial method of synthesizing methanol was produced from coal. After World War II, it was developed to use natural gas, oil refiner and light oil as raw materials. The coal method has declined, and only cheap coal and steel-making industrial areas are still used.
Methanol Synthesis Process
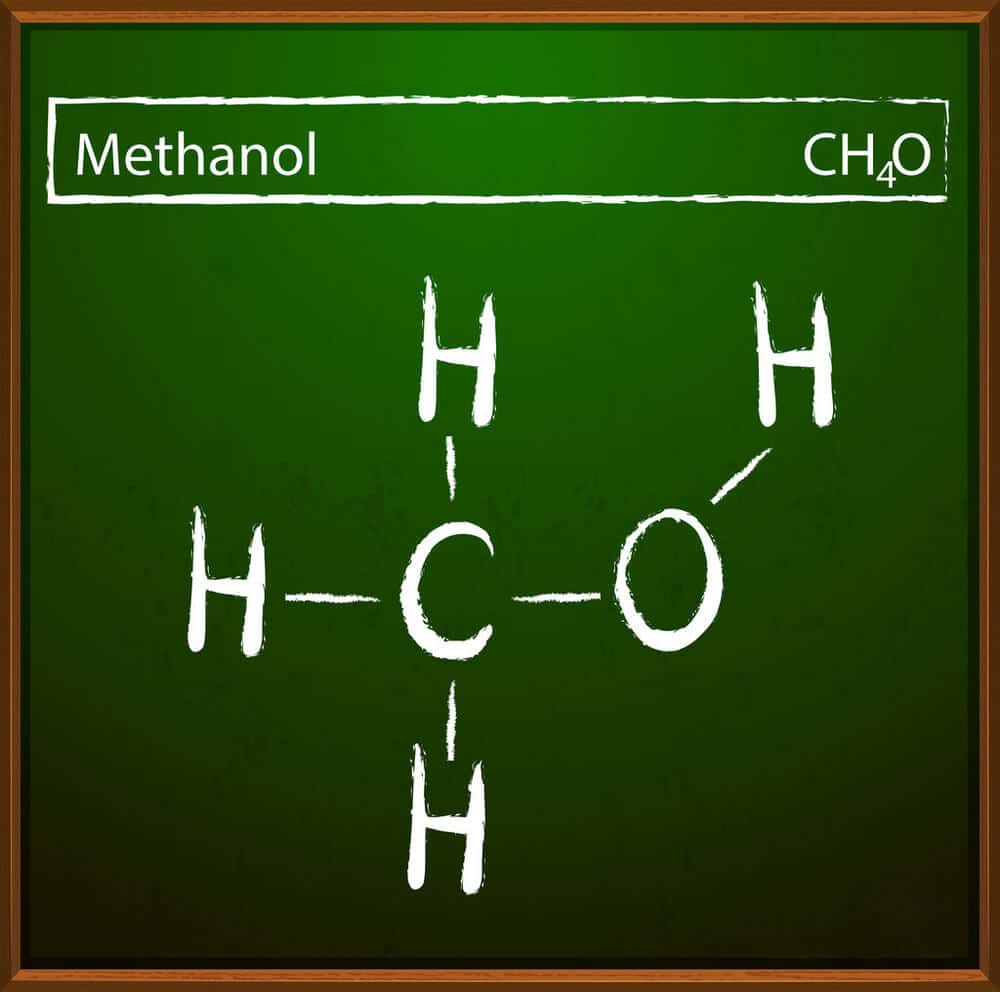
Methanol synthesis from CO2 and H
In the industry, almost all methanol is synthesized by pressurized catalytic hydrogenation of CO and CO2. The typical process includes raw material gas production, raw material gas purification, methanol synthesis, crude methanol rectification and other processes.
Methanol synthesis materials
Natural gas, naphtha, heavy oil, coal and its processed products (coke, coke oven gas), acetylene tail gas, etc. can be used as raw materials for the production of methanol synthesis gas. The steam reforming of natural gas and naphtha needs to be carried out in the reforming furnace with a complex structure and high cost. The converter furnace is equipped with a radiation chamber and a convection chamber, and the hydrocarbon steam gas reforming reaction is carried out at high temperature and in the presence of a catalyst.

The partial oxidation of heavy oil needs to be carried out in a high-temperature gasifier. When solid fuel is used as raw material, intermittent gasification or continuous gasification can be used to produce water gas. The batch gasification method uses air and steam as the gasification agent, and separates the blowing and gas production stages, while the continuous gasification method uses oxygen and steam as the gasification agent, and the process is carried out continuously.
Methanol synthesis catalyst
A variety of catalysts are used in methanol production, such as natural gas and naphtha steam reforming catalysts, and methanol synthesis catalysts. They are susceptible to sulfide and lose their activity, so sulfide must be removed. Gas desulfurization methods can be divided into two categories, one is dry desulfurization, and the other is wet desulfurization. Dry desulfurization equipment is simple, but due to the slow reaction rate, the equipment is relatively large. Wet desulfurization can be divided into three categories: physical absorption method, chemical absorption method and direct oxidation method.
What are the methods for synthesis of methanol?

The methanol synthesis is carried out under high temperature, high pressure and catalyst, which is a typical composite gas-solid phase catalytic reaction process. With the continuous development of methanol synthesis catalyst technology, the general trend is from high pressure to low and medium pressure.
High pressure method
The high-pressure process of synthesizing methanol generally uses zinc-chromium catalyst at 300-400°C and 30MPa of a high temperature and pressure. Since the first successful methanol synthesis by this method in 1923, this method has been continued almost 50 years, differ only in certain design details. For example, heat transfer in the methanol synthesis tower has two types of methods: cold tube continuous heat exchange type and cold shock type multi-stage heat exchange type; the reaction gas flow mode has axial and radial or a mixed type of both, with by-product steam and non-by-product steam flow etc. In recent years, our country has developed the technology of synthesizing methanol on copper-based catalyst under the pressure of 25-27MPa, the content of methanol in the outlet gas is about 4%, and the reaction temperature is 230-290°C.
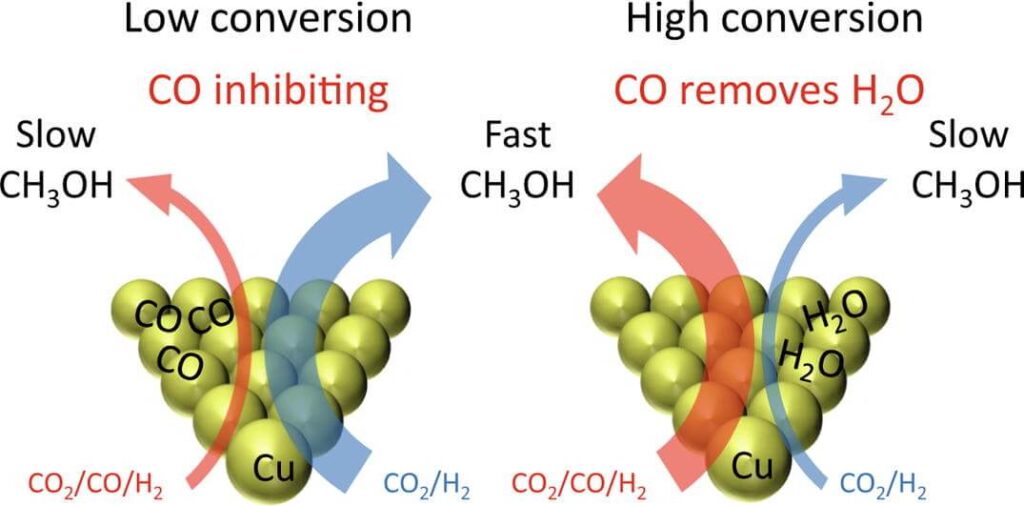
Medium pressure method
The medium-pressure method is further developed on the basis of the low-pressure method. Due to the low operating pressure of the low-pressure method, it will arouse the volume of the equipment is quite large, which is not conducive to the large-scale production of methanol. Therefore, the medium-pressure method for methanol synthesis with about 10MPa pressure has been developed, which can more effectively reduce the cost of building factory area and methanol production.
Low pressure method
The ICl low-pressure methanol method is successfully researched by the British ICl company in 1966, thus breaking the monopoly of the high-pressure method for methanol synthesis, which is a major change in the methanol production process. It uses a 51-1 copper-based catalyst, with the 5MPa synthetic pressure. The synthesis tower used in the ICl method is a hot-wall multi-stage chilling type with a simple structure.
Methanol synthesis application
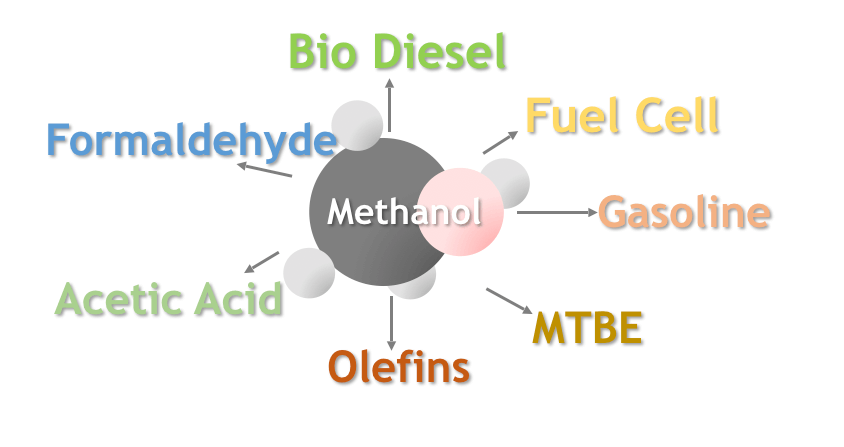
Hydrogen can be used to synthesize methanol. Synthetic methanol is one of the main raw materials in the organic chemical industry and an important petrochemical due to its low production cost and wide range of uses. It can be used for dimethyl phthalate (DMT), methyl methacrylate (MMA), methylamine, polyvinyl alcohol, methyl chloride, acetic acid, etc. In addition, methanol can be used as a solvent and fuel, and hydrogen can be produced if necessary.
What are the raw materials for methanol production?
Methanol synthesis from natural gas
Natural gas is the main raw material for methanol production. The main component of natural gas is methane, and also contains small amounts of other alkanes, alkenes and nitrogen.

There are steam reforming, catalytic partial oxidation, non-catalytic partial oxidation and other methods to produce methanol feed gas from natural gas, among which steam reforming method is the most widely used, and it is carried out in a tube furnace under normal pressure or under pressure. Since the heat of reaction must be supplied from the outside to maintain the required conversion temperature, it is generally realized by burning some kind of fuel gas between the tubes, and the steam for conversion is directly produced on the device by the heat of flue gas and conversion gas.
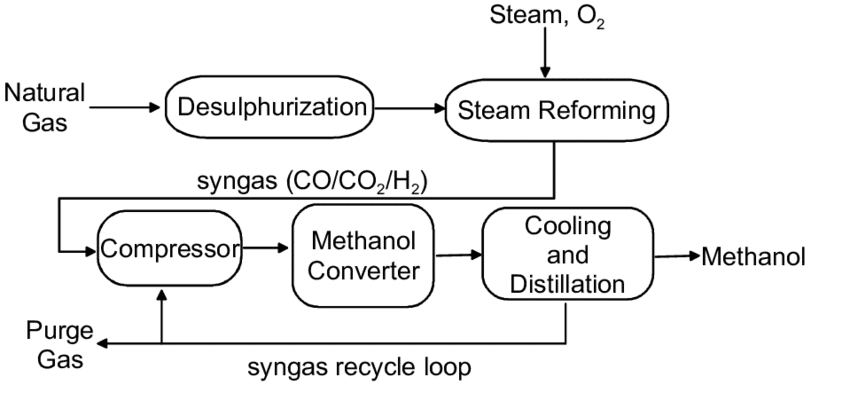
Since the synthesis gas produced by steam reforming of natural gas has excess hydrogen and insufficient carbon monoxide and carbon dioxide, the industrial solution to this problem is to use the steam reforming method with carbon dioxide to achieve a suitable ratio. Carbon dioxide can be supplied externally or by Recovery from reformer flue gas. Another method is the second-stage reforming method using natural gas as raw material, that is, the steam reforming of natural gas is carried out in the first-stage reforming, and only about 1/4 of the methane is reacted. Partial oxidation of natural gas is carried out in the second stage, not only the ratio of the obtained synthesis gas is appropriate, but also the reaction temperature in the second stage is increased to above 800°C. The amount of residual methane can be reduced, and the effective gas components for synthesizing methanol are increased.
Natural gas needs to be purified before entering the steam reformer to remove harmful impurities. The sulfur content of the purified gas is required to be less than 0.1mL/m3, and the converted gas is compressed to synthesize methanol in the synthesis section.
Methanol synthesis from coal and coke

Coal and coke are the main solid fuels for the production of crude raw material gas for methanol. The methanol production process from coal and coke includues fuel gasification, gas desulfurization, shift, decarbonization, methanol synthesis and refining.
The thermal processing of coal and coke with steam and oxygen (or air, oxygen-enriched air) is called solid fuel gasification.
The flammable gas obtained from gasification is generally called coal gas, which is the initial raw material gas for methanol production. The main equipment for gasification is coal gas generator.
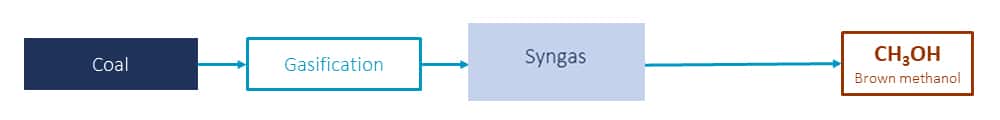
The ratio of hydrogen and carbon in the raw material gas produced from coal and coke is too low, so after gas desulfurization, it needs to go through a shift process to convert excess CO into hydrogen and CO2, and then remove the excess CO2 through the decarburization process. The feed gas is compressed, methanol synthesized and rectified to produce methanol.
Methanol synthesis from heavy oil
Industrially, there are two main types of oil products used to produce methanol: one is naphtha, and the other is heavy oil. The fraction below 220°C obtained by crude oil distillation is called light oil, also known as naphtha. The methods of producing synthesis gas from naphtha include pressurized steam reforming, catalytic partial oxidation, and pressurized non-catalytic partial oxidation, batch catalytic conversion method, etc.
The main method for producing methanol raw gas from naphtha is pressurized steam reforming. It needs to be carried out in a reformer with a complex structure. The reformer is equipped with a radiation chamber and a convection chamber, and the steam reforming reaction of hydrocarbons is carried out at high temperature and with catalyst.
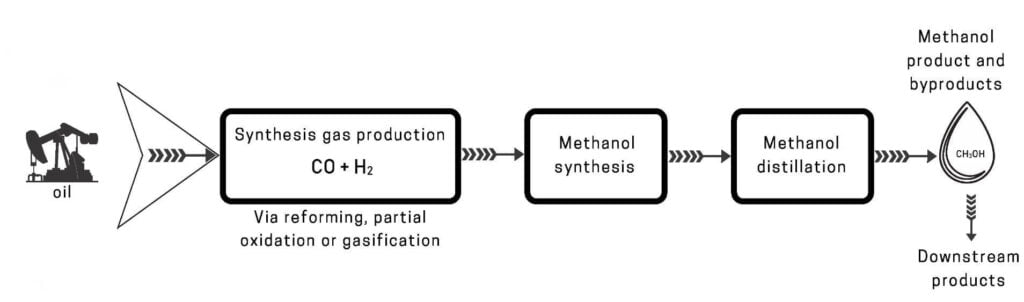
After the naphtha is steam reformed, its composition can just meet the needs of methanol synthesis. There is no need to add carbon dioxide before and after the conversion or to set up a second-stage conversion, and it is not necessary to adjust its composition through conversion and decarbonization.
Heavy oil is a product in the petroleum refining process. According to different refining methods, it can be divided into atmospheric heavy oil, vacuum heavy oil, cracked heavy oil and their mixtures. There are two ways to produce methanol feed gas from heavy oil as raw material: partial oxidation method and pyrolysis method. The pyrolysis method needs to crack the heavy oil in the regenerative furnace at a high temperature above 1400°C. Although oxygen is not needed, the equipment is complicated, the operation is troublesome, and the large amount of carbon black is produced.

Partial oxidation of heavy oil refers to the cmbustion reaction of heavy hydrocarbons and oxygen, and the exothermic reaction causes thermal cracking of some hydrocarbons. The cracked products undergo further oxidation and reforming reactions, and finally obtain H2, CO, and a small amount of CO2. , CH4 to form synthesis gas for methanol synthesis.
The synthesis gas produced by the partial oxidation of heavy oil, due to the high carbon-hydrogen ratio in the raw material heavy oil, and the excessive content of CO and CO2 in the synthesis gas, part of the synthesis gas needs to be transformed. So that CO and water vapor react to form H and CO2, and then removed CO2 to achieve the required composition for methanol synthesis.
The synthesized crude methanol needs to be refined to remove impurities and water to obtain refined methanol.
Co-production of methanol and ammonia

Methanol and ammonia co production is a purification process of synthetic gas, a new process developed to replace the copper ammonia liquid used in many synthetic ammonia productions in my country to remove trace carbon oxides.
The co production condition is to add a set of methanol synthesis device between the outlet of the fifth stage of the compressor and the inlet of the copper washing process, including methanol synthesis tower, circulation machine, water cooler, separator and crude methanol storage tank and other related equipment.
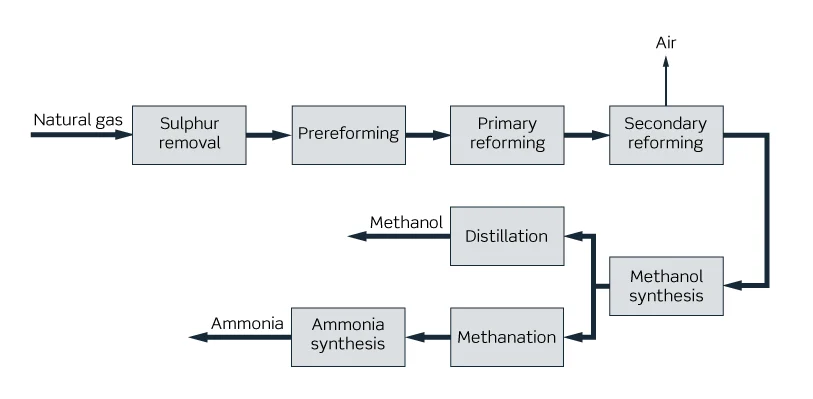
The process flow is that the gas at the five-stage outlet of the compressor is advanced into the methanol synthesis tower. Most of CO and CO2 that were originally removed in the copper washing process react with H in the methanol synthesis tower to form methanol. After the co-production of methanol, CO that enters the copper washing process is significantly reduced, which reduces the copper washing load.
At the same time, CO index in the conversion process can be relaxed appropriately, reducing the steam consumption of the conversion, and CO transported by the cylinders in the first few stages of the compressor becomes effective gas, and the power consumption of the compressor is reduced.
The reduction in energy consumption after co-production of methanol is obvious, which can save 50kW·h of electricity per ton of ammonia and 0.4t of steam, equivalent to 2 million kJ of energy consumption. The process of co-production of methanol and ammonia must pay attention to the fine desulfurization and rectification of raw gas to ensure the service life of the methanol catalyst and the quality of the methanol product.
Which is the advanced catalyst for production of methanol?
Working principle of methanol synthesis
Increasing the pressure and lowering the temperature is conducive to the main reaction to produce methanol. In order to prevent equipment corrosion, the early synthesis method removes CO2 before the reaction. Later, it was found that CO2 can also be used as raw material, so it is not necessary to remove CO2 first.
The key to synthesizing methanol lies in the pressure. In the past, the high-pressure method was used, and the pressure was above 300 atmospheres. In 1966, the low-pressure method was developed, and the pressure was about 50 atmospheres. Later, the medium-pressure method was developed, and the pressure was about 200 atmospheres.
Different methanol synthesis catalyst
| Pressure (atm) | temperature(℃) | catalyst |
| High pressure method | 300~600 320~380 | Chromium zinc catalyst |
| Medium pressure method | 105~300 225~270 | Copper-zinc-chromium-aluminum catalyst |
| Low pressure method | 40~60 200~300 | Copper-zinc catalyst |
Conclusion of methanol synthesis
In conclusion, methanol synthesis is a crucial process for various industries, from fuel to chemical production. The evolution of methanol synthesis has allowed for more efficient and sustainable production methods, making it an increasingly attractive option for industries seeking to reduce their environmental footprint.

At 7Chemtech, we understand the importance of methanol synthesis and its applications. We are committed to providing high-quality solutions to meet the diverse needs of our clients. Our team has the expertise and experience to deliver customized solutions that address the unique challenges of methanol synthesis, from production to application.
If you have any questions or are interested in learning more about our services, please don’t hesitate to contact us. We would be more than happy to discuss how we can help you achieve your goals and contribute to a more sustainable future. Together, let’s continue to push the boundaries of methanol synthesis and create a better world for everyone.

